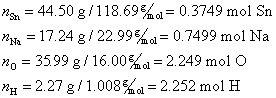
An Example of Percentage Composition
Hydrated sodium stannate has the percentage composition of 44.50% Sn, 17.24% Na, 35.99% O, and 2.27% H. Determine what the correct formula is for hydrated sodium stannate. The correct order of elements is
NaaSnbOc•d H2O
Solution
First, assume 100 g of compound for simplicity. Then calculate the quantity of each element in moles, n.
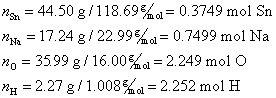
Since the waters of hydration are the only source of hydrogen, it is possible to calculate the quantity of water (in moles) present.
![]()
So far, we can fill in the atom subscripts as
Na0.7499Sn0.3749Oc•1.126 H2O
But we still don't know c.
![]()
Finally, we fill in the subscript on O…
Na0.7499Sn0.3749O1.123•1.126 H2O
But this is a violation of definite proportions, so we normalize to the least-common-multiple (normalize to 1 with 0.3749)
Na2SnO3•3 H2O